Contact enhancing chemicals, cleaners, lubricants for SMS cards
- July 2007
- Background
- Dec 2007
- IEEE September 2000 Study
|
From: Robert B Garner Subject: Study of what is undoubtedly DeoxIT and Re: Two SMS cards coming your way for testing (was Re: Question: Metallurgy of 1960's SMS cards (Re: 1401 restoration, R&D article on Rent rule, etc.) Date: July 17, 2007 at 12:33:48 PM PDT To: Mike Cheponis Cc: ... Mike, Here was PJ Singh's (corrosion processes expert at IBM Poughkeepsie) original advice to me for our situation: > I am not in favor of contact lubricants for gold platings. One > micrometer gold plating with nickel underplate, with high contact > force and no vibration needs no lubricant. Lubricant complicates > connector reliability because one cannot predict the long-term > chemical behavior of lubricants. > If possible, I would just wipe the connectors clean with isopropyl > alcohol But if there's not a nickel layer in our SMS connectors, his advice may be different. Thus, we need to ascertain the gold layer thickness and whether a nickel layer is present. Then I will also ask for WH Abbott's advice at Battelle, (one of the two key corrosion/lubrication researchers of the past 20 years, the other being Morton Antler.) Re: DeoxIT, I'm fairly confident that this article studied DeoxIT* and recommended that it NOT be used as a contact lubricant, because it itself is a corrosive agent! The article debunked the vendor's assertion that "quantum tunneling" was at work for the lubricant and found, consistent with my web search of properties of its active ingredient, polyoxypropylene (or "glycol ether polymer") found: "a mechanism of metal dissolution, ionic conduction and metal re-deposition through electrochemical reactions." The study found it is "very hydroscopic and absorbs moisture readily from the air" and dissolves copper and forms dendrites. Their study found these mechanisms improved conductivity: 1) Ionic conduction increases surface area for conductivity, 2) Dissolution of metal and oxygen (charge carriers) increases conductivity, 3) Ionization at the anode prevents oxide build-up, and 4) Metallic dendrites serve as metallic bridges between mating surfaces. But the article warns that it "may enhance conductivity in the short term, but may lead to degradation of long-term reliability by inducing corrosion failure." It also seemed to reduce the insulation resistance between pins "that operate at different voltages ... that may lead to failure due to high leakage current or even shorting." > So, again, I strongly believe we should NOT be using DeoxIT. (I'll try to contact the authors at Ford Motor.) I'll also send out a summary of what I've learned reading about two dozen original articles on corrosion and lubrication of gold.. > - Robert * I'm fairly certain the article studied DeoxIT because of its (weird) assertion that conduction takes place via "electron tunneling", and it refers to Abbott's/Air Force "Not Recom" rating of a "similar commercial contact enhancer", Stabilant, which also purports "quantum tunneling". For both DeoxIT and Stabilant, they seem to have "considerable solubility of water". From Abbott's paper: "Therefore moisture ingress and possibly an associated pollutant ingress to the metal surface area are responsible for the accelerated corrosion of both steel and porous gold." |
|
In 2007, our 1401 restoration team was debating whether to use contact enhancing chemicals, cleaners, or
lubricants on our SMS connector tabs. In particular, Mike Cheponis had suggested that we might apply “Dexoit”
contact wipes to our SMS card tabs. As I was already investigating metal corrosion for the liquid-cooled IceCube
server project at IBM Almaden Research Lab, I knew the head of IBM’s Materials and Metallurgical Lab in Poughkeepsie, PJ Singh.
I had even visited his remarkable corrosion analysis laboratory and read 28 articles about the nature of metal-contact
corrosion. PJ Singh graciously agreed to analyze two sample of SMS cards. With his advice, and after much discussion,
we decided that it was better not to apply any specialized connector cleaner or lubricant to the SMS tabs.
PJ Singh recommended, given the tabs' robust gold-nickel-copper stack-up, that only isopropyl alcohol should be applied to the SMS tabs.
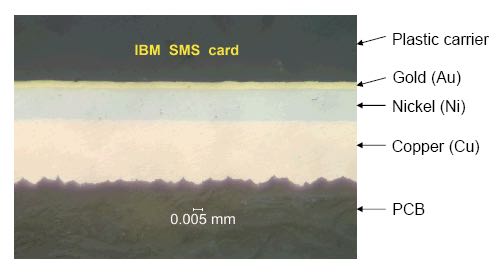
In the report, PJ Singh noted: "I am not in favor of contact lubricants for gold platings. One micrometer gold plating with nickel underplate, with high contact force and no vibration needs no lubricant. Lubricant complicates connector reliability because one cannot predict the long-term chemical behavior of lubricants. Computers in data centers are exposed to 40-50%RH clean air at less than 20 deg C. Generally, metallic corrosion is not a concern below 50% RH.” In a June, 2007 email about our considered use of “Dexoit”, PJ Singh replied: "I am skeptical about the claims folks make about stuff that can clean and improve the reliability of gold contacts. I do believe in lubricants such as Krytox and Nyogel that can reduce wear and fretting corrosion when connectors are subjected to micro vibration. Chemicals, including fluxes, cannot reduce oxides. For example, fluxes remove oxides, not reduce them, by attacking the underlying metal and lifting off the oxides." PJ Singh’s "Energy Dispersive X-ray fluorescence (XRF)" study showed that IBM SMS cards likely have the thickest gold layer ever manufactured: 2.54-microns (100 microinches) thick, with very little to no porosity. From his report:
|
|
From: Robert Garner Date: December 18, 2007 9:25:57 PM PST To: 1401 Restoration Team <1401_team@computerhistory.org> Cc: pdp-1-team@googlegroups.com Subject: IBM report on 1401 SMS card tab metallurgy and corrosion Attachment: SMS_Tabs_IBM_Report_Dec2007.pdf, 1.5 MByte This past summer we were debating and learning about the efficacy of using a corrosion inhibitor/lubricant on the 1401's SMS edge connector gold-plated tabs. At that time I sent two sample SMS cards to IBM Systems and Technology, Poughkeepsie, PJ Singh's Materials & Process Engineering group in order to:
The analysis, based on "energy dispersive X-Ray fluorescense" (EDX), determined the SMS edge connector stackup to be 100 micro-inches (2.54 microns) of Gold over 750 micro-inches of Nickel over Copper. Scanning electron microscopy (SEM) determined that there was no exposed Nickel nor any signs of Gold porosity nor significant surface wear. PJ Singh's conclusion is that there's NO need for a corrosion inhibitor/lubricant. His recommendation is to clean SMS tabs with isopropyl alcohol as necessary. My thanks go to PJ Singh and his team for donating their time and resources to do this timely study for us! - Robert * There are other possibilities, as metallic connectors were a new research field in the late 50's and 1960's. For example, there is an IBM Poughkeepsie 1966 paper that argued for 95.5% Gold / 0.5% Cobalt alloy directly over Copper (no Nickel). ** Our Gold's 2.54-micron thickness is substantially thicker than today's connector gold overcoats, 3/4 to 1 micron. Or even thinner, 0.38 micron or 15 microinches, for 10-year-lifetime, office-grade equipment starting in early 1980's, when standard practice had already dropped Gold thickness to 1.27 microns, or 50 microinches. They don't make 'em like they used to! ;--) p.s. Some more PJ Singh feedback in the report: > In the bygone days, ATT and others thought that connectors needed > many microns of gold plating with nickel underplate. Today 3/4 > micro-meter of gold with nickel underplate does a good job. > I am not in favor of contact lubricants for gold platings. One > micrometer gold plating with nickel underplate, with high contact > force and no vibration needs no lubricant. Lubricant complicates > connector reliability because one cannot predict the long-term > chemical behavior of lubricants. > But maybe we are barking up the wrong tree: Computers in data > centers are exposed to 40-50%RH clean air at less than 20 degrees C. > Generally, metallic corrosion is not a concern below 50% RH.- Robert IBM Almaden Research Center, San Jose, CA Office: 408-927-1739 Mobile: 408-679-0976 robgarn@us.ibm.com |
|
Here is the Sept, 2000 study of what was likely Deoxit in the IEEE Transactions on Component and Packaging Technologies
titled
"Mechanism of Contact Enhancing Chemicals for Electrical Interconnection”:
The paper’s abstract: "Contact enhancing chemicals, the magic fluids which vanquish intermittent contacts, have been in existence for many years. However, their use has been very limited due to the lack of understanding of their conduction mechanism. In this paper, electrochemical experiments were conducted to reveal the mechanism of conduction through the contact enhancing chemicals. The results have invalidated claims by several manufacturers of contact enhancing chemicals that conduction takes place by means of “tunneling.” It has been demonstrated that these novel chemicals function through a rather common conduction mechanism, by serving as an electrolyte for metal dissolution, ionic transport, and metal deposition. Metallurgical examinations have provided direct evidence of the existence of metallic dendrites between mating elements that have been treated with the chemicals, and confirmed the electrochemical reaction mechanism for contact enhancement." Quoting from its conclusions, first describing the nature of metal-to-metal connector contacts… "As a result of the electrochemical reaction [see previous paragraph], metallic dendrites are formed. The growth of the dendrite provides a “bridge” be- tween the mating elements (the wires and the barrel), thus providing a “healing” mechanism for contact enhancement. The formation of the dendrites is the concrete evidence that the conduction mechanism of the contact enhancing material is electro- chemical reaction and ionic conduction. … and how failure occurs: "Compared with oils and greases that are traditionally used on mechanical interfaces for electrical connection, the contact enhancing materials that have limited ionic conductivity and pro- mote dendritic growth have several advantages. As illustrated in Fig. 9, on a microscopic scale, the surface in contact is rather jagged, and metal to metal contact only happens at the high points. As a result, the current densities at these contact points are very high. The high current density at these points can result in localized heating and oxide film build-up, and the oxide film at these points in turn gives rise to high resistance. In addition, physical gaps can also develop between the mating surfaces due to vibration, thermomechanical loading, etc., thereby inducing high contact resistance. The high resistance ultimately leads to the failure of the connection. … and warning about the use of contact enhancing chemicals [my bold]: "It must be cautioned that the enhancement in conductivity comes at the expense of metal corrosion. Corrosion has been a known failure mechanism for contact surfaces. The contact enhancing chemical may enhance conductivity in the short term, but may lead to degradation of long-term reliability by inducing corrosion failure. A previous field and laboratory study [4] has shown that a commercial contact enhancer similar to the subject of current study caused more corrosion than most conventional lubricants.” "In addition, the chemical causes significant decrease in not only the resistance between the mating surfaces of a contact system, but also the insulation resistance between the pins that operate at different voltages. That may lead to failure due to high leakage current or even shorting. Therefore use of such material on connectors with tight spacing between pins, such as edge card connectors for high density electronic circuit boards, can be very dangerous.” One key issue is that Caig Laboratories has not released Deoxit’s ingredients/its secret recipe. All we know is that it’s a volatile organic chemical (VOC). |
return to main page - SMS-Cards